Team: Nucleolar Dynamic Reorganization in action during DNA Repair (Nu-DyRection)
Members













About
One of the most fascinating and relatively unexplored areas in the field of DNA repair is how cells restore their normal functions after completing the processes that eliminate DNA lesions and re-establish DNA sequence continuity. DNA lesions not only impede transcription, replication, and the cell cycle, but may also disrupt the proper positioning of chromatin domains within the nucleus and alter nucleolar organization.
Our team is focused on the molecular mechanisms involved in the restoration of transcriptional activity, particularly the reorganization of nucleolar structure following DNA damage and repair.
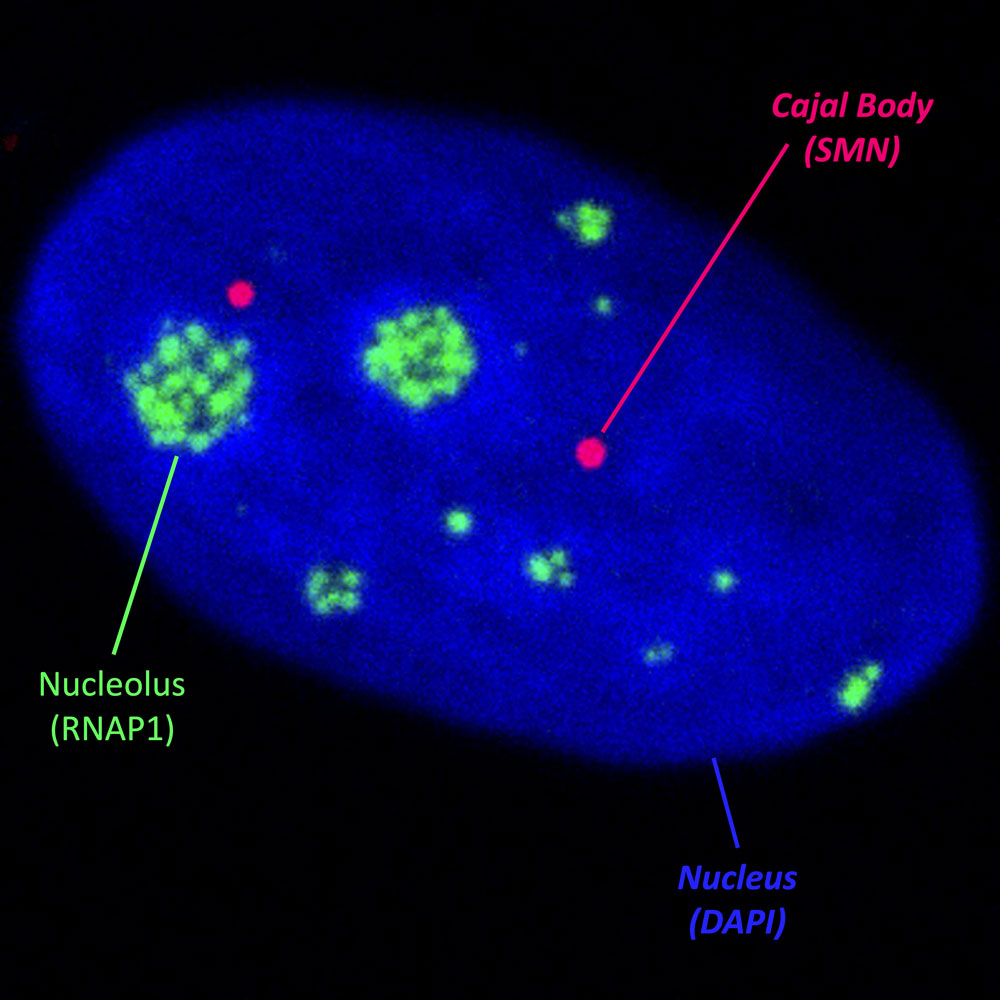
The nucleolus is a membraneless nuclear organelle with a highly structured internal organization. This organization is associated with its various functions in ribosomal biogenesis: transcription of ribosomal DNA (rDNA) by RNA Polymerase 1 (RNAP1) and the early maturation of ribosomal RNA. This highly organized structure can be significantly disrupted by both genotoxic agents and general cellular stress.
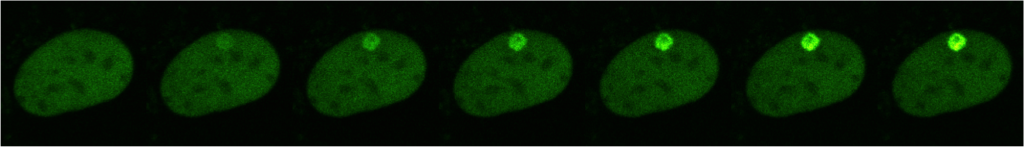
In the last years, we have shown that after a genotoxic stress (UV: Ultraviolet irradiation), RNAP1 and nucleolar DNA are exported to the periphery of the nucleolus. Interestingly, proper nucleolar structure is only restored after the complete repair of all the lesions on the nucleolar DNA, both active and inactive regions. In addition to an efficient repair system, the restoration of normal nucleolar structure after DNA repair completion requires the presence of key proteins.
One such protein is SMN (Survival of Motor Neuron), which is altered in patients suffering from Spinal Muscular Atrophy (SMA). We have found that in absence of SMN, RNAP1 remains at the periphery of the nucleolus after DNA damage repair. Unexpectedly, we observed that SMN shuttles from Cajal bodies (CBs) into the nucleolus after the completion of DNA repair, but before the restoration of nucleolar structure. Together with SMN, other proteins, such as Fibrillarin (FBL), Coilin, Nuclear Myosin 1 (NM1), appear to be also important for this process.
Our group has the following objectives:
- To investigate the dynamic reorganization of nucleoli following stress
- To elucidate the mechanism by which nucleolar homeostasis is restored upon DNA repair completion
- To identify the critical factors governing nucleolar homeostasis during and after DNA Repair
Credit: Lise-Marie Donnio